USA
Hematogenix Laboratory Services
8150 W 185th St. Suite A, Tinley Park, IL 60487
Toll Free: +1 888.436.2861
Telephone: +1 708.444.0444
Fax: +1 708-444-0445
UK
Hematogenix Laboratory Services
BioHub, Mereside, Alderley Park
SK10 4TG, UK
Telephone: +44 1625 238817
Fax: +44 1625 238818
ClientServices@Hematogenix.com
Our Searchable Test Menu
The searchable database is on hold until I can get some assistance from the pathology suite.
Platforms and Partnerships
We need a list of company logos we can show here.
Methods and Departments
At Hematogenix, we are a full-service laboratory with multiple platforms. We accept many different specimen types. Download our up to date specimen requirements.
TECHNOLOGY
Our instrumentation and equipment are compliant with 21 CFR Part 11 requirements. Technologists are trained on GCP requirements.
IMMUNOHISTOCHEMISTRY (IHC)
Hematogenix offers IHC automated instrumentation on platforms that are widely used. Multiple instruments are available to eliminate interruptions to patient recruitment that are being selected by your IHC assay.
FLUORESCENCE IN SITU HYBRIDIZATION (FISH)
Hematogenix offers FISH testing for solid tumor and hematology specimens. Automated slide preparation is available. We enjoy discussing custom probe design for novel targets.
MRNA IN SITU HYBRIDIZATION
mRNA ISH is an alternative to quantitative PCR, with the benefits of morphological detail and standard brightfield microscopy assessment. Hundreds of probes are commercially available, and Hematogenix can create custom probes for new targets or splice variants.
PCR
Hematogenix offers the industry-leading platforms and can validate kit-based mutation assays (BRAF, EGFR). We will work with you to design a validation plan that probes the required limits of assay sensitivity for a specific project or application.
IMAGE ANALYSIS
Hematogenix uses digital pathology and image analysis technologies extensively. Our suite of digital tools enables us to develop objective and reproducible IHC assays for your project and conduct Web conferences with access to biomarker images.
FLOW CYTOMETRY
Our flow cytometry platforms are routinely used to phenotype blood cells for the diagnosis of blood disorders. Consult with us to employ these platforms for biomarker detection for your research and clinical trial needs. Our technicians are experts at various applications including rare event detection.
We accept a broad range of specimen types:
• Peripheral Blood
• Bone Marrow Aspirates
• Liquid Specimens (e.g. Fine Needle Aspirate (FNA), Cerebrospinal Fluid (CSF))
• Fresh Tissue (e.g. Lymph Nodes, Bone Marrow Core Biopsy, Lung, Liver, Breast, Colon, Spleen)
CYTOGENETICS
Our team utilizes fluorescent in situ hybridization (FISH) and other molecular methodologies to rapidly identify targeted chromosomes with suspected abnormalities in interphase cells as well as in metaphases. Our highly trained technical team employs a state-of-the-art high-speed scanner system and networked workstations to provide you valuable images and analyses.
Leading the way in PD-L1 testing
The programmed death 1 (PD-1) pathway has become a major focus of immunotherapy in many cancers. Multiple agents have progressed through clinical development in recent years, including antibodies targeting both PD-1 and its key ligand, programmed death ligand 1 (PD-L1).
We have consistently provided broad commercial access to high-quality PD-L1 testing. It is our continued mission to help our physicians identify the most appropriate treatment options for their patients.
Testing for PD-L1 expression at diagnosis provides actionable information that can be utilized to make treatment decisions across all lines of therapy. Studies show that testing helps to identify how well a patient is likely to respond to PD-1/PD-L1 immunotherapy.
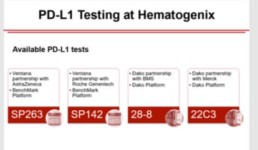
Hematogenix offers all four FDA-approved tests to help our physician clients better understand their patient’s tumor which in turn provides insightful information for their treatment decisions.
• Clinically validated and reproducible
• Proven sensitivity and specificity
• Results available in 3-5 days
Test Name
PD-L1
Specimen Requirements Comments
FFPE Block
Formalin fixed paraffin embedded (FFPE) tumor block
Unstained Slides
If FFPE tissue block is provided as sections, a minimum of 5 unstained sections of 4-5 microns thickness should be provided. Sections must be mounted on positively charged glass slides (e.g. Superfrost Plus Micro Slides)
Fresh Tumor Biopsy
Biopsy should be fixed as close as possible to the time of excision and placed into 10% Neutral Buffered Formalin (NBF) Samples should remain in fixative for 4-48 hours at room temperature
Comments
Storage of samples at site should be kept at a temperature range between 15°C and 25°C.
Immediately ship samples to Hematogenix for further processing into FFPE blocks and testing.
If collected biopsy sample is not sent to Hematogenix on the same day, tissue samples should be transferred to a vial containing 70% ethanol following formalin fixation at site
Pathology Services
No content available

Hematopathology Specialty
No content available
Science
DEEP CLINICAL EXPERTISE
When specialty physicians from around the world need scientifically sound clinical testing, Hematogenix is their first choice. We provide testing in our state-of-the-art laboratory, where we maintain >99% of our testing in house, under one roof. We don’t need to use computer-generated algorithms to diagnose your patients. We know every patient is different. No matter how complex the clinical findings, our clear, concise reports present your patients’ results accurately and definitively. Our team of seasoned pathologists is an extension of your team. Working together, we become valuable advocates in the effort to deliver personalized medicine.
ACCESS TO WORLD-CLASS CLINICAL EXPERTS
Hematogenix’s deep bench of experienced physicians and technologists go way beyond testing and reporting to help you deliver optimal care to your patients. Our clinical experts work side-by-side with you to develop scientifically sound diagnoses and treatment options for every patient.
LESS WAITING FOR YOU AND YOUR PATIENTS
Our focus is you and your patients. We’re not just a service – we’re an exceptional laboratory experience. We will provide you with industry-leading turnaround time, unprecedented quality, and the highest levels of client service from every department and member of our team.
Service
EFFICIENT, EFFECTIVE, AND PERSONAL
At Hematogenix, outstanding science is coupled with exceptional service. You will find our staff, known for their supportive and professional manner, to be experts in their field.
We appreciate that a clinician’s treatment planning protocol often requires more information than our convenient, online reporting options can provide. As a result, our board-certified Hematopathologists are always available for consultation to offer suggestions or additional insight, that will help you choose the correct treatment option for your patient.
We consider it an honor and privilege to be chosen to work side-by-side with you to help you deliver the best care to improve your patient’s quality of life. That’s why, at Hematogenix, you will find a level of personalized, passionate client service not found anywhere else.
The Patient
MORE THAN JUST A CASE
Hematogenix looks beyond the boxes marked on a test request form. What we see each time is a patient waiting eagerly for a diagnostic decision to be made. We work to understand the slight differences each patient’s disease presents and how our diagnosis will impact their treatment.
Our laser focus on appropriate, targeted and clinically useful testing delivers more precise results for you, our physician client. As a result, you can be confident that the experience you have with our staff and the diagnosis you receive from our laboratory will be what you need from a group of laboratory professionals to help you deliver the best treatment plan and produce an optimal outcome your patient deserves.
Hematogenix PD-L1 Published Studies:
Agreement Between Programmed Cell Death Ligand-1 Diagnostic Assays Across Multiple Protein Expression Cut-Offs in Non-Small Cell Lung Cancer
Concordance of tumor cell (TC) and immune cell (IC) staining with Ventana SP142, Ventana SP263, Dako 28-8 and Dako 22C3 PD-L1 IHC tests in NSCLC patient samples
Concordance of Tumour and Immune Cell staining with SP142, SP263, 22C3 and 28-8 PD-L1 tests across different Cancer Types, European Congress Pathology, September 2017 (NEED LINK)
A Study of PD-L1 Diagnostic Assay Concordance In Urothelial Carcinoma (NEED LINK)
USA
Hematogenix Laboratory Services
8150 W 185th St. Suite A, Tinley Park, IL 60487
Toll Free: +1 888.436.2861
Telephone: +1 708.444.0444
Fax: +1 708-444-0445
UK
Hematogenix Laboratory Services
BioHub, Mereside, Alderley Park
SK10 4TG, UK
Telephone: +44 1625 238817
Fax: +44 1625 238818
ClientServices@Hematogenix.com

